Recommendations for Moderna Booster Dose Modified
Posted on January 13, 2022 by Kari Everson
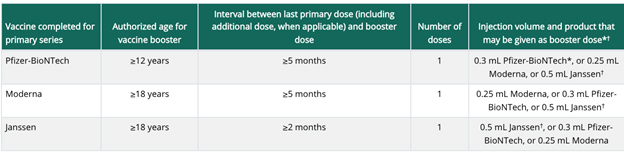
On Jan. 7, the Food and Drug Administration (FDA) modified their recommendations for when a booster dose of the Moderna vaccine should be administered, and the CDC has published updated information. The booster recommendations are the same for both Pfizer and Moderna with this update.
The booster doses for individuals who received a two-dose primary series of either mRNA vaccine, Pfizer or Moderna, should be administered after five months. Recommendations for the Johnson & Johnson vaccine remain unchanged. Those who received the one-dose J&J vaccine should receive a booster dose after two months.
The CDC guidance has a table available for reference.
Updated Emergency Use Authorization (EUA) fact sheets and other information related to the Moderna vaccine can be found on the FDA’s Moderna vaccine site.
For questions concerning COVID-19 vaccination, please contact Kari Everson.
Comments
Add a comment
Members must sign in to comment
You must be a member to comment on this article. If you are already a member, please log in. Not a member? Learn how to join »

No one has commented on this article yet. Please post a comment below.